Chemistry primer
Summary
An understanding of basic chemistry is essential to understand plants and their interactions with their environment. Elements are substances that cannot be broken down further by chemical processes. Atoms are the smallest chemically indivisible units of elements, and are made up of nuclei, composed of protons and neutrons, and electrons, arranged in orbitals. Atoms react by donating or sharing electrons to form bonds, respectively ionic and covalent bonds. Atoms that are bonded together form molecules. Atoms and molecules that carry electrical charge are called ions.
Chemistry is the science that studies what the world around us is made of (“matter”), and tries to understand its properties and behaviour. In particular, it looks into the different types of matter that exist at very small scales and how these interact with each other. These interactions (“chemical reactions”) are fundamental to everything in the universe, including plants (and soil). At some point in our journey to understand plants we need an understanding of the basics of chemistry. This article, and the next few articles, will try to provide a refresher of just enough chemistry (and a little bit of physics) for the purposes of Getting Dirty.
Electrical charge
There are four fundamental forces in the universe: “gravity” (which causes objects to be drawn to each other based on their “mass”, a measure of how much matter they are composed of), “electromagnetism”, and two “nuclear forces” (strong and weak), which will not concern us in Getting Dirty. We are all familiar with gravity, since it is the force that causes things to fall towards the surface of the earth.
We are mostly familiar with electromagnetism from school experiments with magnets. These have two distinct ends (called the North and South “poles”) and if the same poles from two magnets are brought together, there is a repulsive force; whereas if two different poles are brought together, they are attracted to each other.
Electricity and magnetism are intimately connected with each other (this is how electric motors work, but we don’t need to go into the details of this connection). So in a similar way to the poles of a magnet, every piece of matter in the universe carries an “electrical charge”. This can be positive, negative or neutral. If two substances with the same charge are brought together, they repel each other; and if they have opposite charge, they are attracted to each other. The strength of the charge on each substance governs the strength of the force between them (this force also reduces the further apart the substances are). This force is the electromagnetic force.
Elements, atoms, ions and molecules
Everything around us is made up of “elements”, substances that cannot be broken down further by chemical processes (we will explain what we mean by this in just a moment). The smallest chemically indivisible particle of an element is called an “atom”.
Atoms consist of a “nucleus”, made up of electrically neutral particles called “neutrons” and positively charged particles called “protons”, surrounded by negatively charged particles called “electrons”, which are arranged in a hierarchical structure of “orbitals” (sometimes called “electron shells”). Atoms are electrically neutral, and the electrical charge on the proton (+1) and the electron (-1) are of the same magnitude, so the number of electrons and the number of protons are the same. This number is called the “atomic number” of the element.
A simple picture of a chlorine (Cl) atom, showing the nucleus in the centre, and electrons arranged in orbitals around this.
Two atoms with the same atomic number are indistinguishable from each other in terms of how they interact with other particles (it is possible that they may have differing numbers of neutrons, in which case they are said to be “isotopes” of each other, and can be distinguished by their mass, even though not by their interactions). On the other hand, substances with different atomic number interact in distinctly different ways to each other.
Chemistry is concerned with the properties of substances and their interactions, so in chemistry, the atomic number of a substance is fundamental. Chemical processes are those which do not affect the atomic number of the substances that are interacting. Under extreme conditions of temperature and pressure, substances can sometimes be forced to change their atomic number. These are “nuclear processes”, and belong within physics, not within chemistry. They are also not relevant for us in our journey to understand plants.
The periodic table of the elements
All elements can be listed in what is known as the “periodic table”. All elements in the same column of the table share similar characteristics in terms of their interactions with other elements. This is because they have the same number of electrons in their outermost orbital, and it is this number of electrons that governs how “reactive” the element is; in other words, how readily it interacts with other substances (the true picture is significantly more complicated than this, but would require a detour into quantum mechanics, and is beyond our scope). Each element in the periodic table is given a one or two letter symbol, such as C for carbon, or N for nitrogen.
Periodic table of the elements, here coloured to show which elements are most relevant to plants
Chemical reactions
Atoms react with each other in one of two ways: by donating electrons, and by sharing electrons. This creates a “bond” between the two atoms, which now behave together as a single particle, called a “molecule”. When the bond is formed by an electron being donated, it is called an “ionic bond”. When the electron is shared, it is a “covalent bond”.
Each orbital in an atom can only hold a fixed number of electrons; when the innermost orbital is full, further electrons must be held in the next orbital outwards, and so on. The number of electrons in an atom’s outermost orbital is a key driver of how reactive that element is; in a sense, atoms want to have full electron orbitals and reach with other elements to achieve this.
The elements on the far right of the periodic table (called the “noble gases”) have a full outermost orbital and are highly unreactive; they are often described as inert, but can be persuaded to react under the right conditions. Those on the far left have only one electron in their outermost orbital, and those in the second right column are only missing one electron. Both groups are highly reactive.
Ionic bonding
For example, an atom of sodium (Na, on the far left column, so with one outer electron) reacts with an atom of chlorine (Cl, on the second right column, so missing one outer electron) by donating an electron. In this way, the outermost orbital of sodium now effectively has no electrons (and hence the next orbital is complete), and the outermost orbital of chlorine is completed.
The sodium particle now has one less electron than proton and hence has a net positive charge. Similarly, the chlorine particle now has one more electron than proton and hence has a net negative charge. Atoms must be electrically neutral, so these particles are now known as “ions” (electrically charged molecules are also called ions). Positive ions are called “cations” and negative ions are called “anions”.
Since the sodium ion is positively charged and the chlorine ion is negatively charged, there is now an electromagnetic attraction between them, which holds them together. This is the ionic bond.
An atom of sodium (Na) and an atom of chlorine (Cl) share an electron to form an ionic bond.
Substances that react by forming ionic bonds typically go on to form crystals, regularly arranged lattices of ions joined by ionic bonds. Sodium chloride (table salt, the substance formed in the above reaction) does this, with each sodium ion surrounded by six chlorine ions (one each above, below, to the left, to the right, in front, and behind); and similarly each chlorine ion surrounded by six sodium ions.
When ionic substances are placed in water, their crystal lattice breaks apart and they dissolve, with their ions floating freely in the water. This is called a (ionic) solution.
Covalent bonding
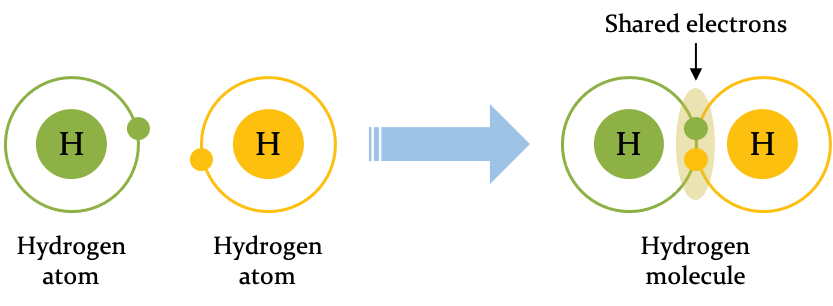
Hydrogen (at the top left of the periodic table, with just a single electron and a single proton) gives an example of covalent bonding. The outermost orbital of a hydrogen atom has capacity for two electrons. Two hydrogen atoms can share their electrons so that the electron of each completes the outermost orbital of the other. The outcome is a molecule of hydrogen gas (written H₂), and the sharing of the electron is a covalent bond.
Two atoms of hydrogen share an electron to form a covalent bond.
Multiple bonds
Atoms can share or donate more than one electron in a molecule. If two electrons are shared or donated between the same two atoms, this is a “double” bond; if three, a “triple” bond.
Notation
Ions are written using the symbol for the corresponding atom, together with the electric charge as a superscript. Examples include Na⁺, Cl⁻, Fe²⁺, and Fe³⁺ (some atoms have more than one ionic form, with the outermost orbital still incomplete, but closer to being full than in the atom). Molecules are written using the component atoms, with the number of each present written as a subscript. Examples include H₂O, NaCl, NH₄, and CO₂.