More chemistry
Summary
Chemistry can be divided into two main areas of study: organic chemistry, which studies molecules based on carbon, and inorganic chemistry, which studies non-carbon-based molecules. Organic molecules have carbon skeletons based on chains of bonds between carbon atoms, with other elements then attached to these skeletons. Plants take in inorganic nutrients from the soil and assemble these into complex organic compounds, the most important of which are carbohydrates, proteins, lipids and nucleic acids. In addition, plants produce secondary organic compounds used for defence, signalling and many other purposes.
Within all of chemistry, one element plays a unique role; that element is carbon. Carbon is so special that the whole study of chemistry is divided into two parts based on whether or not the compounds being studied contain carbon or not. Compounds that contain carbon are called “organic” compounds, and their study is “organic chemistry”; those that do not are called “inorganic” compounds, and their study is “inorganic chemistry”. But why is carbon so important?
A carbon atom has four electrons in its outermost orbital, out of a possible eight
The answer lies in the ability of carbon atoms to form an incredibly diverse range of structures by bonding with other carbon atoms. A carbon atom has four electrons in its outermost orbital, out of a possible eight. Each of these electrons can bond with other carbon atoms, which in turn can bond with yet more carbon atoms (some of these bonds may be double, or even triple, bonds), to form a “carbon skeleton”. These carbon-carbon bonds are strong and stable, not easily broken apart in chemical reactions. The remaining electrons, those that do not bond with carbon atoms, can then form bonds with other, non-carbon, atoms.
Carbon atoms can form long chains of bonds with each other to create a huge variety of structures
Approximately two hundred million compounds of carbon have been described and indexed (Source: Wikipedia), with this representing only a tiny fraction of the theoretically possible carbon compounds. Carbon compounds include one of the softest materials known (graphite) as well as one of the hardest (diamond). They occur in all known lifeforms, which is why they are known as organic compounds - and why they are important in understanding how plants work.
Inorganic chemistry is important to understand plants for a different reason. The mineral nutrients that plants take up from the soil are inorganic compounds (or simple non-carbon ions). Plants process these inorganic compounds, stripping off their non-carbon atoms, and incorporating these into complex organic compounds that they manufacture within their cells. These organic compounds include a bewildering diversity of molecules, which are categorised by organic chemists into well known groups such as sugars, starches, fats, proteins and so on.
Organic chemistry is phenomenally complex and well beyond the scope of Getting Dirty. For our purposes, all we really need to understand is that most of the substances produced by plants, including those that they use for their critical processes (such as photosynthesis) are organic compounds, and that these take the form of carbon skeletons with other non-carbon elements added to their structure. At the end of this article, I’ve included more detail on two important types of organic compound, carbohydrates and proteins, but this is purely for the interested reader.
Common types of organic compounds produced by plants
Plants produce many millions of different organic compounds, many of whose purpose is neither known nor understood. These may be used for defence against pests, pathogens and herbivores; to attract pollinators or animals to disperse seeds; to send signals to other plants or to bacteria and fungi in the soil; or for a myriad of other purposes.
At the same time, many groups of organic compounds are reasonably well understood, and it is worth knowing the names and roles of these within a plant. The four most important such groups are the following:
‘Carbohydrates’ are a broad group of relatively simple organic compounds, including both sugars and starches, as well as cellulose, a key component of plants’ cell walls. Carbohydrates are used by plants to store and transport energy, and as structural materials
‘Proteins’ are complex molecules assembled from simpler structures called “amino acids” (which themselves play other roles in a plant, in particular in the assembly of other, more complex substances). A huge diversity of proteins, each with different chemical and physical structures (the shape of a protein molecule, determined by how it folds up on itself, is often key to its role) is used by plants: for structure; for selectively transporting other molecules to where they are needed; for regulating the pace of chemical reactions in cells; for sending signals and instructions between cells; and for many more critical functions.
‘Nucleic acids’, RNA and DNA, store and execute the blueprint and instructions for a living plant, as well as encoding the genetic information that is passed down to the plant’s offspring. One way in which they do this is by controlling the production/degradation and activity of proteins in the plant.
‘Lipids’, including fats, waxes and oils, are used by plants to form a semi-permeable membrane within their cells, allowing the plant to control which substances can move in or out of its cells (using proteins as gatekeepers). Lipids are also used to store energy (for example in seeds, many of which contain energy-rich oils), and in signalling within the plant.
Carbohydrates and lipids are made of only three elements: carbon, hydrogen and oxygen. Proteins and amino acids also include nitrogen within their structures. And nucleic acids contain both nitrogen and phosphorus in addition to carbon, hydrogen and oxygen. The remaining critical plant macronutrient, potassium, is not used structurally in these compounds, but instead plays a key role in the management and transport of energy, water, nutrients and carbohydrates within the plant, helping to regulate the rate and activity of critical processes, such as photosynthesis.
Some more technical details for the interested reader
Carbohydrates
Carbohydrates are one of the most fundamental groups of organic molecules. Compounds in this group have both oxygen and hydrogen atoms attached to their carbon skeleton, and include both sugars and starches. The carbon skeleton of a carbohydrate can be a simple chain of carbon atoms, or it can branch.
Oxygen atoms have six electrons in their outermost orbital (out of a possible eight), so are capable of forming two covalent bonds with other atoms. This means that an oxygen atom can form a double bond with a carbon atom (forming a “carbonyl group” C=O), or it can form a single bond with a carbon atom and another with a hydrogen atom (forming a “hydroxyl group” C-O-H). A simple sugar usually has a carbonyl group attached to one of its carbon atoms, and a single hydroxyl group attached to each of the others.
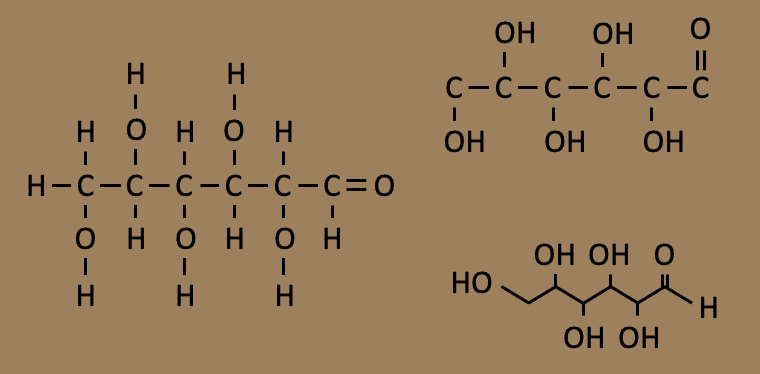
Three different ways of illustrating a glucose molecule. On the left, showing all of the bonds. On the top right, excluding the carbon-to-hydrogen bonds, and on the bottom right, showing only the carbonyl and hydroxyl groups attached to the carbon skeleton. This last illustration clearly shows the key elements of glucose’s structure.
An oxygen atom in a carbohydrate can also bond to another carbon atom. This means that two (or more) carbon skeletons can be joined together by an oxygen atom sitting in the middle, forming a “glycosidic bond” between them. This fact is used to sort carbohydrates into three groups:
“Monosaccharides”, which have a single carbon skeleton, to which hydrogen and oxygen atoms are attached. Some common monosaccharides are glucose, fructose and galactose;
“Disaccharides”, which have two carbon skeletons, again with hydrogen and oxygen atoms attached to each, and with the two skeletons joined through an oxygen atom. Some common disaccharides are sucrose, lactose and maltose;
“Polysaccharides”, which have more than two carbon skeletons joined through oxygen atoms, all with hydrogen and oxygen atoms attached. Polysaccharides can be incredibly complex, with structures that branch in many different directions. As such, names for common polysaccharides usually encompass many different molecular structures that share common properties, rather than referring to a single molecular structure. Starch and cellulose are examples of polysaccharides.
Monosaccharides and disaccharides are commonly referred to as “sugars (sometimes “simple sugars” and “double sugars” respectively).
The carbon-carbon bonds in a carbon skeleton are strong and stable, and difficult to break down. However, glycosidic bonds are weaker, and can be gradually broken down, allowing polysaccharides to be used by plants to “store” sugars.
Proteins
Two other groups that can be attached to a carbon skeleton are “amino” groups and “carboxyl” groups. An amino group consists of a nitrogen atom and two hydrogen atoms. A carboxyl group consists of both a carbonyl and a hydroxyl group attached to the same carbon atom.
An amino group on the left; and a carboxyl group on the right
An organic compound that contains both amino and carboxyl groups is called an “amino acid”. Over 500 amino acids exists in nature, with 22 of these being essential for life.
Amino acids can bond with each other through the amino group of one reacting with the carboxyl group of the other. The hydroxyl (-OH) group of the carboxyl group and one of the hydrogen atoms in the amino group together form a water molecule. The remaining constituents link together to form a “peptide bond” (O=C-N-H).
Large groups of amino acids can bond with each other through peptide bonds to form very large, very complex molecules. These molecules are called “proteins” and play a very wide range of roles in plants, including facilitating essential chemical reactions, forming structural parts of plant cells, sending signals throughout the plant, and transporting molecules across cell membranes.
The essential nature of proteins is part of why plants cannot thrive without adequate nitrogen. Nitrogen must be incorporated into amino acids, which are then manufactured into proteins. And proteins are used everywhere that a plant is growing or repairing damage to its cells. Nitrogen (and phosphorus) is also a component of the nucleic acids, RNA and DNA, which store and implement a plant’s blueprint for life.
Other organic compounds
The chemistry of other organic compounds critical to plants, such as nucleic acids and lipids, is significantly more technical. I find this fascinating, but had to take a decision to stop here as this article is already getting both long and in depth. Perhaps a topic to revisit one day.