Movement in plants
Summary
Plants rely on a number of processes to move fluids, including water, nutrients and carbohydrates, through their tissues to where they are needed by the plant. Passive transport processes, such as diffusion, osmosis, and mass flow, occur naturally due to the tendency of fluids to flow from areas where a particular physical property, such as pressure, solute concentration or electrical charge, is high to areas where it is low. Active transport processes require the plant to expend energy to move substances in the opposite direction. Diffusion is essential to gaseous exchange; osmosis is essential to the absorption and regulation of water in the plant; and mass flow is used to transport fluids in bulk through the xylem and phloem. Active transport processes are used in many places, in particular in the absorption of nutrients from the soil into the plant.
Unlike animals, plants do not have internal pumps (our hearts and lungs) to move fluids (liquids and gases) to where they are needed. Instead, plants must make direct use of physics and chemistry to transport water, nutrients, gases, and other molecules to their destinations.
Key to understanding plants’ transport processes is the idea of a “gradient”, whereby a particular physical property has a higher level in one place and a lower level in another. In the same way as a ball rolls down a hill (from a high point to a low point), fluids naturally tend to flow down gradients, which may be due to pressure, electrical charge, or how concentrated a solution is, for example. Transport processes that move down a gradient are called “passive transport” processes. Those that move up a gradient are called “active transport” processes, and need a plant to expend energy (generated through respiration from carbohydrates produced in photosynthesis).
The three most important passive transport processes are diffusion, osmosis and mass flow. A further passive transport process is capillary action (see this article)
Diffusion
Fluids flow down a “concentration gradient” by means of “diffusion”. A concentration gradient just means that there is more of a fluid (in the sense of more molecules of that fluid) in one place than in another.
Fluid flow along a concentration gradient. In the box on the left, there are significantly more molecules (a higher concentration) at one end than the other. Over time, the molecules spread out into the space nearby (middle box). Left long enough, the molecules become evenly spread across the whole space (right hand box). The result is that the fluid has flowed from one end to the other until the concentrations are the same, and there is no longer a concentration gradient.
Molecules are constantly moving about (the speed at which they move depends on the amount of energy they have, something that we perceive as temperature at our normal scale instead of the microscopic scale we are considering now). When there are lots of molecules close together, they tend to bounce off one another. However, if there is some empty space nearby, it is more likely that some of the molecules will move into it. As time passes, more molecules move into the empty space. This reduces the concentration (the average number of molecules in a fixed volume of space) in the original place, and increases the concentration in what was the empty space.
Left long enough, the molecules will spread themselves out more or less evenly across the whole space. When this happens, the concentration of the fluid becomes equal throughout the whole space, and the concentration gradient disappears. This doesn’t mean that the fluid has stopped moving; just that the movement in one direction is balanced out by the movement in the other (a situation known as “equilibrium”).
The net result of this movement is that some of the fluid moves from areas of high concentration to areas of low concentration (i.e. it flows down the concentration gradient) until the concentration equalises. This is the (net) movement of a fluid by diffusion.
Diffusion of a fluid in another fluid
In the real world, fluids are not surrounded by empty space. Instead, there are other fluids around them. The principle however remains the same: fluids move from areas of high concentration to areas of low concentration.
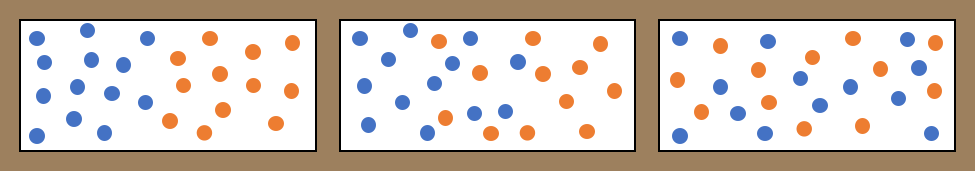
Diffusion of a fluid in another fluid. In the box on the left, there is a concentration gradient of the blue molecules from left to right, and a concentration gradient of the orange molecules from right to left. Over time, the molecules intermingle (middle box). Left long enough, the molecules become evenly mixed over the whole space (right hand box). The result is that the blue fluid has flowed from left to right, and the orange fluid from right to left, and both concentration gradients have vanished.
When two (or more) fluids occupy the same space, it is possible that each fluid has a concentration gradient (or that one or more of the fluids is already evenly spread out and the other(s) diffuse through it). In this case, each fluid will diffuse through the other(s) until their concentrations equalise throughout the space.
Rather than it being more likely that some of the molecules of a fluid will move into empty space, it is now more likely that some will move into an area with fewer of their own kind (simply because molecules’ movements are random, and there are fewer possible arrangements of the molecules where they all stay together than there are where they become spread out; hence there is a higher chance at any time that the arrangement will become more spread out). As a result, there is again a net movement from areas of higher concentration to areas of lower concentration; or a flow down the concentration gradient.
Diffusion in gases and liquids
The distinguishing characteristic of a gas versus a liquid is that its molecules are much more dispersed. In a liquid, molecules are close together, and their movements are constrained by each other (in a solid, this constraint is much stronger, resulting in motions of molecules largely consisting of vibrations in place, which is why solids are rigid and liquids flow). In a gas, molecules are spread out, and can move much more freely.
The result of this is that diffusion happens much faster in gases than in liquids. This is particularly true when the liquid is water, as in the case in plants; water molecules are attracted to each other by hydrogen bonds, meaning that it is much more difficult for other fluids to diffuse through water, than it is through air, for example. Typically, diffusion in air happens approximately 10,000 times as fast as in water.
As a result, plants use diffusion primarily for the transport of gases into and out of their tissues, bringing carbon dioxide in and releasing oxygen during photosynthesis; and bringing oxygen in and releasing carbon dioxide during respiration. This “gaseous exchange” is of critical importance for plants, so diffusion is an important passive transport process.
Osmosis
Plant cells protect themselves from the outside world through two key structures, an outer cell wall, and within it, a cell membrane (see this article). However, plants need to allow fluids to pass in and out of their cells as part of their function. One of the key processes to enable this is “osmosis”.
Cell walls are generally flexible, and their structure is porous at very small scales, allowing small molecules (such as water and nutrients) to flow through them, but restricting the flow of larger molecules (such as viruses and bacteria) into the cell. It should be noted, however, that some specialised cells (such as xylem, or cells in the bark of trees) have secondary cell walls that are both rigid and waterproof.
Cell membranes are formed of a double layer of lipids (molecules such as fats, waxes and oils, see this article). These lipids have the property of forming a dense barrier on both sides of the membrane that is difficult for other molecules to penetrate. Some molecules, such as water, are able to pass through the membrane directly, squeezing between the lipids on each side (albeit a very slow process). The membrane however also has specialised structures (made of proteins) that selectively permit certain molecules to pass through. One of these structures (called an “aquaporin”), allows the transmission of water molecules.
Osmosis across a cell membrane. The cell membrane permits water molecules (blue dots) to pass through, but not dissolved molecules (orange dots). Water passes across the membrane to equalise the solute concentration on either side.
Osmosis happens when a substance (the “solute”) is dissolved in water on either side of a cell membrane, but at different concentrations. In other words, on one side of the membrane, there are more molecules of the solute compared to water molecules than is the case on the other side of the membrane (the diagram above may help to visualise this - on the left hand side there are the same number of water molecules both above and below the membrane, but far more solute molecules below the membrane; so the solution is more concentrated on the lower side).
Osmosis is sometimes described as the diffusion of water across a membrane due to a concentration gradient of water molecules in the solute (since a higher ratio of solute molecules to water molecules is the same thing as a lower ratio of water molecules to solute molecules). However, osmosis results in more water molecules (as well as more solute molecules) being on one side of the membrane than the other, so this explanation is clearly incorrect.
The correct explanation of osmosis depends on two things: the fact that the membrane permits water molecules to pass, but not solute molecules; and the intermolecular forces between the water molecules and the solute molecules (see this article).
Each time a molecule of the solute approaches the aquaporin, it is unable to pass through, and its momentum causes it to be repelled (think of it as bouncing off the membrane). The forces between the solute molecule and nearby water molecules then result in a small force pushing the combined fluid away from the entrance to the aquaporin. On the side of the membrane with more solute molecules, this force is stronger than on the side with fewer solute molecules (because it is more likely that a solute molecule will bounce off the membrance).
This means that, close to the aquaporin, the number of water molecules is lower on the side of the membrane with a higher solute concentration (more solute molecules, and hence a stronger force pushing the fluid away from the aquaporin), than it is on the side with a lower solute concentration (fewer solute molecules, and hence a weaker force pushing the fluid away from the aquaporin). In other words, close to the aquaporin, there is a concentration gradient of water molecules allowing water to diffuse away from the more dilute solution towards the more concentrated solution.
Once water molecules have passed through the aquaporin, the same mechanism makes it less likely that they will flow back to the more dilute solution. As a result, there is a net flow of water molecules through the cell membrane until the solution is equally concentrated on either side. This is osmosis, and it can result in water flowing from an area of lower pressure into an area of higher pressure, if the solute concentration in the higher pressure area is sufficiently high.
The above explanation may be a little complex on first reading, but it is worth taking the time to understand, as osmosis is a critical passive transport process in plants (and indeed, in animals), allowing them to absorb water from the soil, and to regulate the amount of water in their cells (and hence to regulate the concentration of different solutes in their cells). This in turn allows plants to maintain the rigidity of their tissues (caused by the cell membrane pushing against the cell wall), and to control the extent and pace of gaseous exchange (by opening and closing pores in their leaves).
Mass flow
Diffusion and osmosis are both understood at the molecular level. The third passive transport process, “mass flow”, can be understood easily at a more familiar scale. Whereas diffusion and osmosis involve the movement of molecules along a concentration gradient within a fluid, resulting in a net movement of the fluid overall, mass flow involves the movement of the fluid overall along a pressure gradient.
Water pressure is something we are all familiar with from our home plumbing, and consists of water being subject to compressing forces. Water is kept under pressure in the mains water system (with the pressure generated either by gravity, from water held in elevated water towers, or by pumps), and when it is released (for example by opening a tap), water flows rapidly from the high pressure mains into the low pressure air outside the tap.
Water pressure can also be negative, if instead of being compressed, the water is put under tension, for example when we suck on a straw immersed in a glass of water. There is a limit to the amount of negative pressure that water can be placed under before the tension resolves itself by “cavitation”, the formation of small cavities in the liquid, filled with water vapour.
A pressure gradient results in a flow of a fluid from high to low pressure.
If there is a pressure gradient between two volumes of water in contact with each other, the water under higher pressure is subject to stronger compressive forces than that under lower pressure. These compressive forces are pushing on the water molecules in all directions, except that of the pressure gradient. As the water molecules push against each other in response to these forces, they are able to push more strongly in the direction of the pressure gradient (since the push back is weaker from the lower pressure volumes towards the higher pressure volume). This imbalance of forces results in a net flow of water down the pressure gradient until the pressures are equalised. This is mass flow.
The situation is actually a little bit more complicated, since another force may be acting at the same time; the force of gravity. If the pressure gradient is horizontal, the above description is accurate. If the pressure gradient is vertical, then gravity will act from top to bottom, either reinforcing or countering the force created by the pressure difference. If the pressure gradient is at any other angle, the effect of gravity will be part way between the two.
It may be worth noting that in a vertical column of water, gravity does not cause water to flow downwards because it places water at the bottom of the column under greater pressure than that at the top; the resulting pressure gradient generates an upward force that counterbalances gravity, allowing the fluid to remain stationary.
Because mass flow is a movement of a fluid as a whole, rather than of molecules within the fluid (as in the case of diffusion or osmosis), it is able to carry any substance dissolved or suspended in the water along with it. As such, mass flow is the passive transport force used by plants to move water (with dissolved nutrients) through their xylem, and to move carbohydrates and other substances through their phloem.
Water potential
As can be seen from the above discussion, there are many different factors that may result in the passive movement of water through a plant. Rather than always considering each of them separately, it is sometimes useful to consider the aggregate effect of all of them together.
To do this, scientists have developed a concept called “water potential”, which plays the same role in the overall movement of water as pressure does in mass flow. Pressure can be thought of as creating a potential force that only comes into play when there is a pressure gradient. Similarly, potential forces can be defined for osmosis, gravity and capillary action. Water potential is the sum of these potential forces, and a water potential gradient results in a flow of water down this gradient.
Active transport
Unfortunately, plants often need to move substances in the opposite direction to a gradient. An example is in the absorption of nutrients from the soil. Nutrients are present in the soil in low concentrations, but are held in plants’ roots in higher concentrations. If left to passive transport, nutrients would flow out of the plant into the soil, to the detriment of the plant. Plants rely on “active transport” processes to counter this effect.
Active transport involves significant complexity, beyond the scope of Getting Dirty. As such, we will only give a very high level description of one active transport process, to provide an idea of how this works in practice.
In addition to aquaporins, cell membranes contain other “transport proteins”. Some of these proteins can take more than one different shape, depending on what other molecules they bind themselves to. In one type of active transport process, a plant cell releases a hydrogen ion into the space outside a cell membrane. This hydrogen ion binds with a transport protein, causing it to change shape into a new configuration, which is capable of binding to a specific molecule, such as a nutrient.
When the transport protein binds with a nutrient molecule, it simultaneously releases the hydrogen ion into the cell interior, and changes shape again to the original configuration, which is capable of binding with another hydrogen ion. When the transport protein binds with a hydrogen ion, it releases the nutrient molecule into the cell interior, and again changes shape to the nutrient binding configuration. Thus for each hydrogen ion emitted by the cell, a nutrient molecule can be absorbed into the cell.
This “proton pump” mechanism (so called because a hydrogen ion is just a proton) requires the plant to expend energy to produce the hydrogen ion. This energy, generated through respiration using sugars generated through photosynthesis, is essential to move a substance in the opposite direction to a gradient (in exactly the same way as a person must expend energy to climb up a hill).